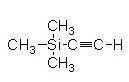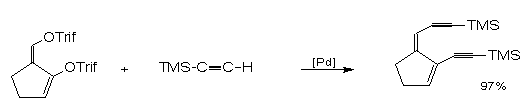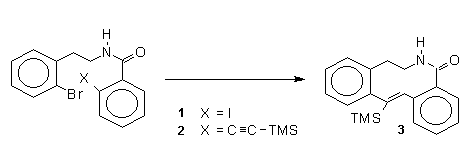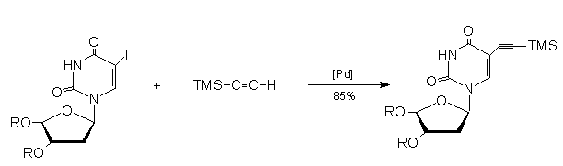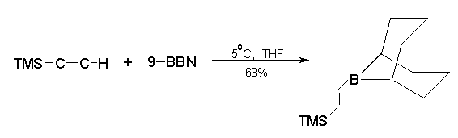Methods
Trimethylsilylacetylene has found tremendous utility in
the ethynylation of aromatic, heteroaromatics and
olefinic compounds via the palladium catalyzed coupling
with halides and triflates. It has become a significant
method for the introduction of a terminal acetylene into
organic molecules. Other synthetic uses of TMSA
include alkylation via Grignard or alkali metal
reagents, hydroboration and hydrosilation.
Palladium Catalyzed Trimethylsilylethynylations
Takahashi1
and Austin2 reported the synthesis of ortho and para
substituted phenylacetylenes by this method. Lau3
disclosed the synthesis of meta substituted derivatives,
and Volhardt et al.4 made hexaethynylbenzene from
hexabromobenzene. In an interesting route to substituted
indoles, Yamanaka et al5 used an o-bromophenylcarbamate
ester to couple with TMSA en route to
2-substituted indoles. In 1986 Chen and Yang6 found the
trifluoromethanesulfonate group to be a good leaving
group in this reaction. Robbins and Barrre vide infra
reported the synthesis of 5-ethynyluracil derivatives,
potential anti-cancer and anti-viral agents. Hirota7
reported the use of the triflate leaving group in this
class of compounds. More recently Suffert8 employed the
same methodology in the synthesis of ene-diyne
precursors.
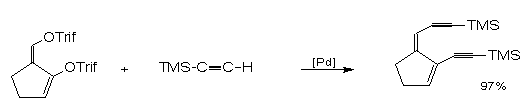
Castedo
et al.9 described a novel method for a
macrocyclization process en route to isoquinoline
alkaloids. Starting from amide (1) which
was chemoselectively condensed with TMSA to give
(2) in 90 % yield. Dropwise addition of
tributyltin hydride in the presence of AIBN gave the
vinylsilane in 60 % yield that was converted successfuly
in a series of steps to macrocyclic lactam (3).
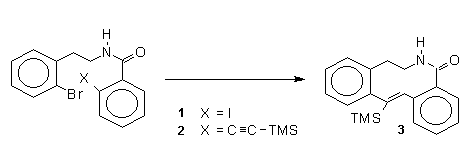
Diphenylketene on reaction with TMSA and other terminal
alkynes in the presence of Pd(PPh3)4 gives
dissubsituted alkynes in good yield (10).
In the field of molecular electronics Suffert and
Ziessel11 reacted TMSA in the presence of palladium
catalyst with a series of mono- and dihalo-
pyridines,bipyridines and other polyimine to give after
desilylation the corresopnding ethynyl polyimines. the
molecules have been shown to present high electrical
conductivity when they are complexed with
tetracyanoquinone and cationic copper.
Trimethylsilylacetylene finds utility in building
polyethynyl aromatics that makes them attractive as
thermoset precursors and another synthetic sequence,
vide infra, utilizes trimethylsilyacetylene in the
synthesis of thiophene based conjugated oligomers of
interest in materials science.
Anti-tumor Agents - Ene-diynes
Ene-diynes
are chemicals made by soil bacteria that mimic
antibiotics. They have been shown to be powerful
anti-cancer agents attacking tumor cells with high
selectivity. Nicolaou12 described them as "Molecular
warheads with triggering devices." More simply when
they penetrate a cancer cell, the molecule is activated
to produce a radical that will selectively kill the
cancer cell by scission of its DNA.
The facile rearrangement by which these 3-ene-1,5-diynes
are transformed into an arene-1,4-diradical(Bergman
Rearrangement)13 has brought a new focus to this area of
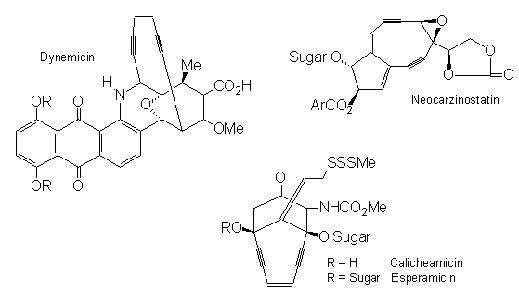
research. The discovery of an array of these natural
toxins such as Neocarzinostatin, Calichemicin,
Esperamicin, and Dynemicin has elicited considerable
interest in the synthetic challenge presented by their
rare structure and their ability to fight cancer cells.
The key step in any synthetic route to the ene-diynes
skeleton relies on placing the alkyne tethers around the
olefinic section of the molecule. Properly designed
stable synthetic analogs with triple bonds that can
produce DNA strand scission are being sought in many
laboratories around the world. In any syntheses,
regardless of strategy, TMSA is playing an important
role.
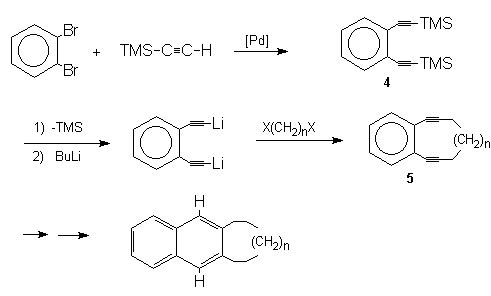
Semmelhack
et al.14 reacted TMSA with o-dibromobenzene
to give the 1,2-bis(trimethylsilylethynyl)benzene (4)
by the method of Hagihara1 and Austin2. This was further
subjected to desilylation in order to set the stage for
connecting the two acetylene tethers via
deprotonation with BuLi and subsequent alkylation with
an a,w-dihalide to produce the cyclic ene-diyne (5)
required for the tandem Bergman-Radical cyclization,
which has become a significant tool due to its ability
to bring about cyclization of substituted ene-diyne
systems.
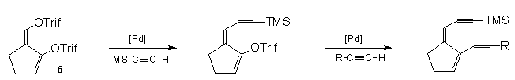
Sufferts
group targeted synthetic analogs of neocarzinostatin8.
Central to their methodology 8,15,16 is Palladium
mediated stereo selective coupling of TMSA with the
isomerically pure bis(enoltriflate) (6) derived
from 2-formycyclopentanone. In this regard they
discovered that the exocyclic triflate ethynylates at a
much faster rate than the endocyclic counterpart8
enabling them to tailor the alkyne groupings that are
suitable for their neocarzinostatin models.
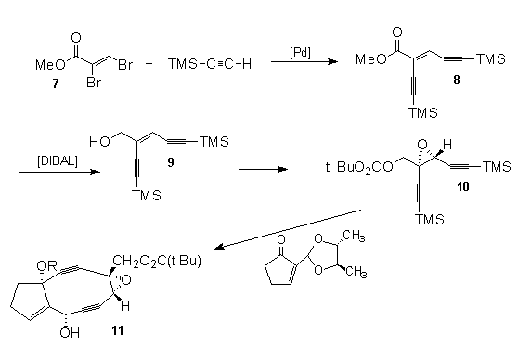
An elegant
enantioselective approach to the highly functionalized
epoxy diyne analog of the neocarzinastin core was
reported by Meyers et al.17 Again TMSA coupling
is central in this synthetic scenario, where it was
coupled with (Z)-ethyl 2,3-dibromopropenoate (7)
under modified Cachi18 conditions to afford the required
(Z)-enediyne (8) in 88% yield. Reduction of the
ester group, followed by selective desilylation with
sodium trimethoxyborohydride gave pure monosilylated
enediyne (9). Catalytic Sharpless19 asymmetric
epoxidation of (9) and subsequent in situ
esterification with pivaloyl chloride produced the
required enediyne (10) that set the stage for the
condensation with the masked 2-formylcyclopentenone
resulting, after several transformations, in the
construction of the highly strained carbocyclic enediyne
(11) with the unusual assembly of the required
group substitution along its periphery
Magnus's
group21,22 approach to dynemicin takes into
consideration the quinoline sub unit of dynemicin (13)
to anchor the ene-diyne portion of the molecule. They
have made several important analogs that rely on h2
dicobalt hexacarbony complex to initiate the final ring
closure. Thus the ene-diynyl Grignard (14),
generated from (12) after desilylation, was
reacted with the quinoline (13) to give the
carboethoxy addition product (15). That was
treated, after removal of the THP, with Co2(CO)8 to give
the cyclic ene-diyne (16).
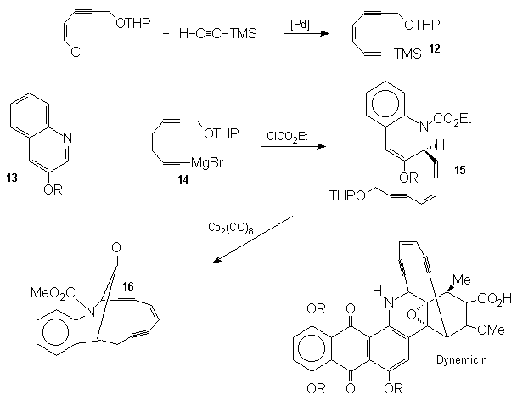
Nicolaou23, among the models that he chose as analogs
to Dynemicin, made the one represented here from the
ethynyl quinoline (17). Palladium assisted
coupling with the vinyl chloride (18), followed
by desilylation resulted in the formation of (19).
Ring closure effected by treatment with LDA anchored
the tether to the quinoline ring to give the targeted
dynemicin analog (20).
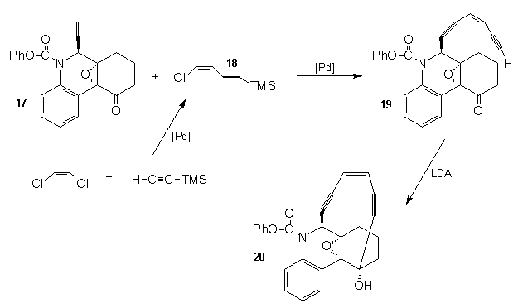
By
incorporating a benzene or a naphthalene ring into the
olefin portion of (18), Nicolaou24 was able to
make a series of dynemicin models in which he modified
some sites around the quinoline core that allowed him to
monitor and better understand the ene-diyne cascade.
Anti-tumor Agents -Taxol
The
synthesis of Taxol has been a considerable synthetic
challenge with its novel and rare ring system. A
convergent intramolecular Diels-Alder approach to the
highly functionalized tricyclo[9.3.1.03,8] pentadecene
nucleus of the taxol skeleton was recently reported by
Fallis and Lu25. Their strategy takes into
consideration the importance of building the right
tethers around the A ring of taxol, that ultimately
allows for a successful Diels-Alder reaction and
therefore permits the construction the Taxol nucleus.
The diene-aldehyde (21) was reacted with lithium
trimethylsilylacetylide to give the alcohol (22).
Removal of the TMS group, followed by Des-Martin
periodinate oxidation gave the required dienophile (23),
which enabled the cyclization to produce the tricyclic
ring system (24).
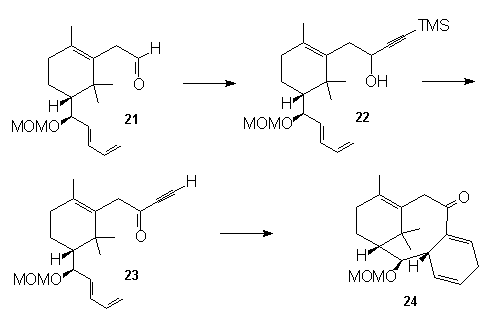
Anti-tumor, Anti-viral Agents - Nucleosides
Robins and
Barr26 reported the synthesis of 5-ethynyl-3',5'-di-O-P-tolulyl-2'-deoxyuridine
by removal of the TMS group that was introduced in the
palladium catalyzed coupling of TMSA with the
5-Iodouridine. This acetylene group is readily subject
to functional elaboration. Moreover,
5-ethynyl-2'-deoxyuridine has potent biological activity
, its 5'-monophosphate is a powerful thymidylate
synthetase inhibitor and it is a potential antiviral
agent.
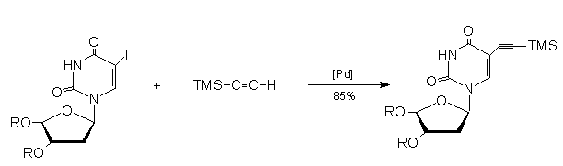
Tanaka
et al.27 produced 6-ethynyl-2'-deoxyuridine by this
reaction on the blocked starting material. More
recently Gillies and Slocock 28 in used this process to
produce other alkynyl antiviral agents.
Anti-Tumor Agents -Tetrahydorofolic acids
Taylor
29,30et al. demonstrated that in the area
of anti-tumor tetrahydrofolic acid derivatives, TMSA
serves a pivotal role as a masked ethylene group in
bridging a gaunine and a glutamate via palladium
assisted carbon-carbon coupling to produce key
intermediates. Thus, 2-pivaloyl-6-bromo-5-deazapterin is
coupled with (4-ethynylbenzoyl)glutamte to give
5,10-dideaza-5,6,7,8-tetrahydrofolic acid(DDATHF), after
redution of the ethynyl group28. Another DDATHF analogue
with promising clinical results as a thymidate synthase
inhibitor was made using TMSA , thus subjecting
2-pivaloyl-7-iodo-deazaguanine (25) to a
palladium catalyzed carbon-carbon coupling with dimethyl
(4-ethynylbenzoyl)glutamate (26), gave the
disubstituted acetylene(27). Selective
hydrogenation of the triple bond was then smoothly
accomplished to furnish the target DDATHF analog after
sponification of the pivaloyl protecting group at N-2.
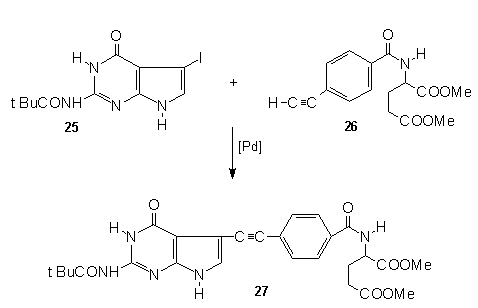
Other Palladium Catalyzed Trimethylsilylethynylations
Trimethylsilylacetylene plays an important role in new
emerging technologies in the field of materials research
.
Whitesides and Neenan31 described the preparation of
highly cross-linked polyethynyl solids containing high
atom frations of carbon. These are made by the
condensation of TMSA with the corresponding poly
haloaromatics. Other researchers also made polyethynyl
aromatics that on thermolysis produce glassy materials
that exhibit high char yield up to 90%. This makes them
attractive as thermoset precursors.32,33 Note that some
of these highly ethynylated compounds have been reported
to decompose violently in the pure state.
Tour et al.34 have developed a synthetic sequence
that utilizes trimethylsilyacetylene as a building block
to allow the rapid formation of conjugated oligomers of
known length and composition for use in nanofabrication
technology.
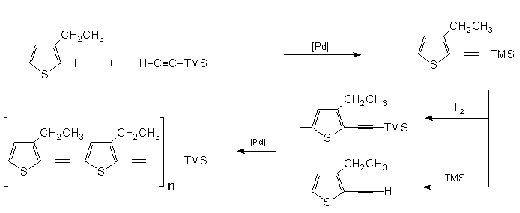
Nye35
developed a polyimide that incorporates an acetylene
spacer unit between the aromatic diimide through the
usual palladium catalyzed trimethylsilylethynylation as
shown in the scheme below. The acetylene spacer resulted
in a rigid polymer with improved characteristics in
terms of chemical resistance and thermal stability. This
polymer has considerable interest in materials research.
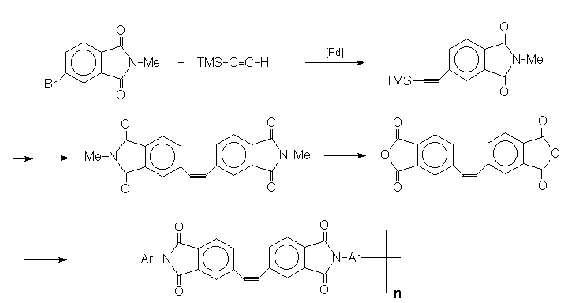
Addition Reactions
Trimethylsilylacetylene like many other acetylenes are
capable of adding many groups a cross the triple bond.
These are very well documented in the literature.
Unlike many hydroborating reagents, 9-BBN adds to TMSA
in high regioselectivity to produce a powerful
dienophile (28) with greater stability than
vinyl-9-BBN36.
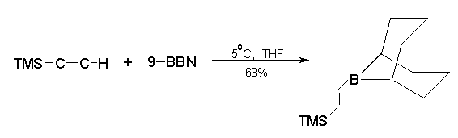
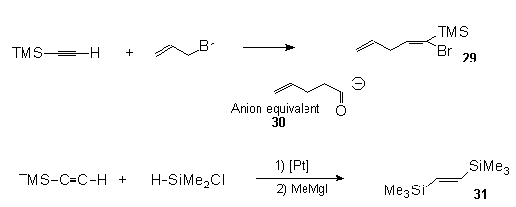
Yamaguchi
et al.37 found that TMSA can undergo a facile
regiospecific bromoallylation in the presence of
dibromobis(benzonitrile)palladium to give the vinyl
silane (29) in 94 % yield. This can easily serve
as a 4-pentenoyl anion equivalent (30), a
valuable synthetic tool. Trans-1,2-bis(trimethylsilyl)ethylene(31)
is made by direct hydrosilylation of TMSA with
chlorodimethylsilane in the presence of Speier's
catalyst followed by addition of CH3MgI38
Alkylation
Lithium
trimethylsilylacetylide is a powerful nucleophile that
has been added to alkyl halide39, epoxides40,aldeydes41
and ketones42. Trimethylsilylethynyl magnesium halides
add to propargyl tosylate43 to yield
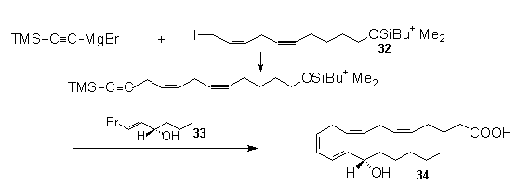
1-trimethylsilyl-1,4-pentadiyne . Nicolaou et al.44
employed this principle effectively in their synthesis
of 15(S)-HETE (34) that was shown to be an
inhibitor of 5-lipoxygenase. The acetylide was
sequentially reacted with two differernt alkylating
agents. The first was intodroduced through the allylic
iodide (32) , while the second subsitution was
brought about via palladium catalyzed coupling with the
vinyl bromide (33) following reduction of the
internal alkyne and removal of the TMS group. Fianlly
reduction of the ethynyl group provided the desired cis
double bond.
Chiral Syntheses
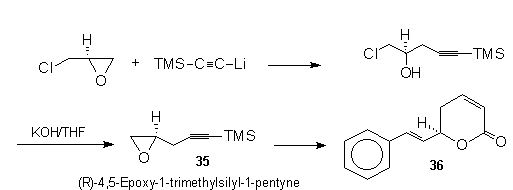
Takano
et al.45 prepared the novel epoxyacetylene ,
(R)-4,5-epoxy-1-trimethylsilyl-1-pentyne (35),
from optially active epichlorohydrin and lithium
trimethylsilylacetylide. This chiral epoxyacetylene
provides a general starting point for a variety of
efficient enantioselective syntheses. An example being
(+)-goniothalamin (36).
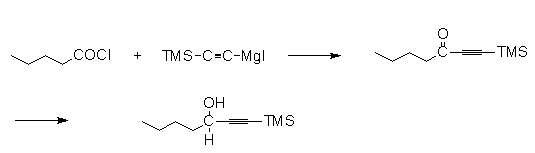
Colobert
and Gentet used TMSA in acylation reactions providing
ketones that can be selectively reduced with an Alpine
Borane to the optically active alcohol46.
Summary
TMSA is
being used in many areas in the forefront of research.
Pharmaceutical research is looking at anti-cancer and
anti-viral agents as well as the development of new
chiral synthetic methods. Materials science is using
TMSA in the development of new polymers for various
applications. Petra Research, Inc. is a leader is
specialty acetylenes and offers bulk quantities of
Trimethylsilylacetylene.
References
1. Takahashi, S. et
al., Synthesis, 627 (1980).
2. Austin, W. B. et al., J. Org. Chem.,
46, 2280, (1981).
3. Lau, K. and Boschan, R., U.S. Patt. App.
197300, 15 Oct 1980.
4. Volhardt, K., et al., Angew. Chem.,
Int. Ed. Engl., 25 268. (1986)
5. Yamanaka, H., et al., Hetrocycles,
24, 31, (1986).
6. Chen, Q. and Yang, Z., Tetrahedron Lett.,
27, 1171, (1986).
7. Hirota, K. et al., Hetrocycles,
26, 355, (1987).
8. Suffert, J. and Bruckner, R., Tetrahedron Lett.,32,
1453, 1991
9. Castedo, T. et al., Tetrahedron Lett.,
33, 5653 (1992).
10. Mitsudo, T., Kadokura, M. and Watanabe,Y.,
Tetrahedron Lett 26, 3697
(1985)
11. Suffert, J. and Ziessel, R., Tetrahedron Lett.,
32, 757 (1991)
12. Nicolaou, K.C. et al., Science, 256,
1172, (1992)
13. Jones, R. and Bergman, R.G., J. Am. Chem. Soc.,
94, 660, (1972)
14. Semmelhack, F.M., Neu, T. and Foubelo, F.,
Tetrahedron Lett., 33, 3277, 1992
15. Brukner, R., Sceuplein, S.W. and Suffert, J.Tetrahedron
Lett., 32, 1449, 1991
16. Scheupein, S.W., Harms, K., Bruckner and R.,
Suffert, J., Chem.Ber, 125, 271,
1992
17. Meyers, A.G., Harrington, P.M. and Kuo, E.Y., J.
Am.Chem. Soc, 113, 694, 1991
18. S. Cachi, E.Morera, G. Ortar, Synthesis,
320, 1986
19. Sharpless, K.B. et al., J.Am.Chem.Soc.,109,
5765, 1987.
21. Magnus, P. and Frott, S.M., J.Chem.Soc.,
Chem.Comm., 544 (1991)
22. Magnus, P. et al., J. Am. Chem. Soc.,
114, 2544 (1992)
23. Nicolaou, K.C. et al., J.Am.Chem.Soc.,
112, 7416 (1990).
24. Nicolaou, K.C. et al., J.Am.Chem.Soc,
113, 9878 (1991).
25. Lu, Y.F. and Fallis, A..G. Tetrahedron Lett.,
34, , (1993)
26. M.J. Robins , P.J. Barr, Tetrahedron Lett.,
22, 421, 1981
27. Tanaka, Can J. Chem 64, 1560
(1986)
28. Gillies, I and Slocock, K.A., Eur. Pat. Appl.,
465164, January 8, 1992
29. Taylor E.C. and Wong, G.S.W., J. Org. Chem.,
54, 3618 (1989).
30. Taylor, D. et al., J.Med.Chem., 35,
4450 (1992).
31. Neenan , T.X. and Whitesides, G.M., J.Org. Chem.,
53, 2489 (1988).
32. Stephens, E.B. and Tour, J.M., Adv. Mater.,
4, 570 (1992).
33. Ibid, Macromolecules, in press.
34. Tour, J.M. and Schumm, J.S., Polymer Reprints,
in press
35. Nye,S.A., J. PolymerChem. Part A., 28,
2633 (1990).
36. Singleton D.A. and Martinez, J.P. Tetrahedron
Lett 32, 7365 (1991)
37. R.Yamaguchi, R. et al., Syn. Comm., 12,
1027 (1982)
38. Birkofer, L. and Keuhn, T. Chem. Ber. 111,
3119 (1978)
39. Castelano, A.L. and Krantz, A., J.Am.Chem.Soc,
109, 3491 (1987).
40. Magnus, P. and Becker, P., ibid, 109,7495
(1987).
41a. Trost, B.M. and Matsuda, K., ibid., 110,
5233, (1988).
41b. Fevig, T.L., Elliiot,
R.L. and Curran, D.P., ibid, 110, 5064
(1988).
41c. Linderman, R.J. and
Suhr, Y., J. Org. Chem, 53, 1569
1988).
42a. Liebeskind, L.S.,
Mitchell, D. and Foster, B.S. J.Am .Chem. Soc,
109, 7908( 1987).
42b. E.J. Corey, et al.,
ibid, 109, 4717 (1987).
43. Verkruijsse,
H.D. and Hasselaar M., Synthesis, 1979,
292
44. Nicolaou, K.C. et al., J.Chem. Soc.,
Chem. Commun. 1985, 1580.
45. Takano, S. et al., Tetrahedron:
Asymmetry, 3, 853, 1992.
46. Colobert, F. and Gentet, J.P., Tetrahedron Lett
26 2779 (1985)